Under Whose Watch Did The Ranbaxy Fraud Happen?
Japanese companies are not known for washing their dirty linen in public, but I suppose pleading guilty to civil and criminal charges in the world's largest and most lucrative pharmaceuticals market, the USA, and agreeing to pay a $500 million fine, could cause some abnormal behavior.
On 22nd May, one week after Ranbaxy, the company it bought in 2008 for $4.6 billion, pleaded guilty with the US FDA, Daiichi Sankyo, said the following in a press release:
"Daiichi Sankyo believes that certain former shareholders of Ranbaxy concealed and misrepresented critical information concerning the U.S. DOJ and FDA investigations. Daiichi Sankyo is currently pursuing its available legal remedies and cannot comment further on the subject at this time."
As corporate press releases go, that was about as close as one can get to a cruise missile. And in case there was any doubt who they were referring to, the answer was provided the very next.
Again, via a press release. In the erstwhile owners of Ranbaxy, the "Singh Family" comprising Malvinder and Shivinder Singh, retorted:
"Daiichi Sankyo's allegations of concealment and misrepresentation are false and baseless.
[...]
The belated suggestion, made years after the fact, that information was concealed from and/or misrepresented to Daichii Sankyo is false and designed to divert attention away from Daiichi Sankyo's own failures to protect itself and its shareholders in the negotiations and agreement with the Singh family shareholders of Ranbaxy.
[...]
It was recently reported that Ranbaxy had entered into a settlement agreement with the US FDA and DOJ in relation to their investigations and that under that settlement agreement, Ranbaxy agreed to pay large penalties to the US FDA and DOJ. The decision to enter into that settlement agreement was made by Ranbaxy and had nothing to do with the Singh family which was not even consulted by Daiichi Sankyo/Ranbaxy."
In essence, Ranbaxy's previous owners were saying that they had not hidden anything from Daiichi Sankyo during the due diligence prior to June 2008 (when the sale was inked). And, more importantly, they seemed to imply that the decisions to plead guilty before the FDA and agree to pay $500 million was not something they would have recommended, had Daiichi Sankyo consulted them.
A somewhat similar press release went out from Ranbaxy on 15th February, 2007, a day after the FDA raided their offices in the US, infamously called "The Great Valentine's Day Raid" by its employees. It said:
"Ranbaxy Inc., said today that federal officials conducted a search at its New Jersey offices on Feb 14, 2007.
Ranbaxy said that this action has come as a surprise. The company is not aware of any wrongdoing."
That was nearly one and a half years before Daiichi Sankyo would buy Ranbaxy.
To unravel the sequence of events, the best resource would be Katherine Eban's fantastic feature story, "Dirty medicine", in Fortune magazine. Pieced together using reportage spread over years, dozens of conversations and multiple Freedom of Information (FOI is the US equivalent of India's Right to Information act) requests, the story is quite simply, superlative. If you haven't read it till now, do so immediately.
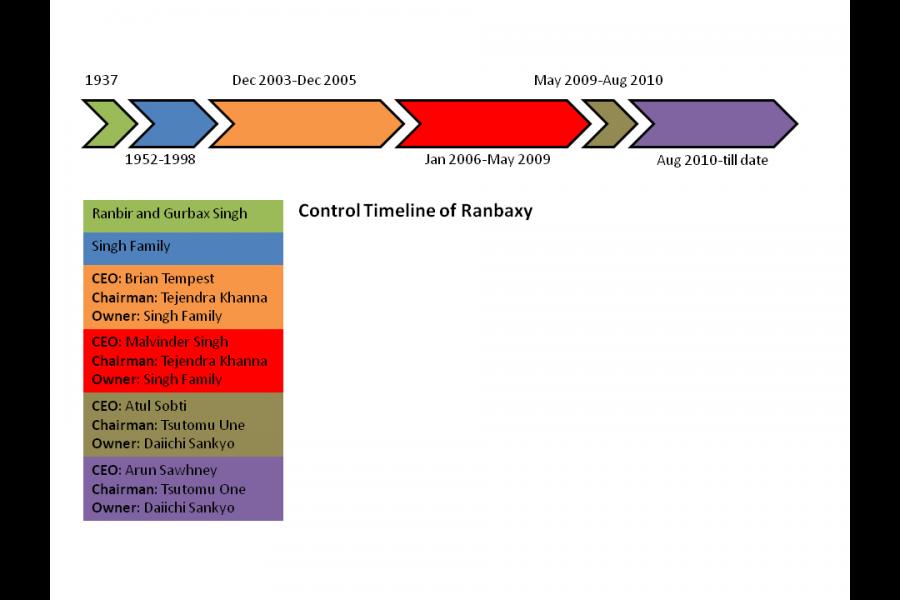
In order to establish the timeline of events, I drew out the key dates and events from Eban's story and correlated it with data on who was CEO, Chairman of the Board and majority owner. Ranbaxy pleading guilty on May 13th was the culmination of a series of lapses and outright fraud, much of which seems to have started years before Daiichi Sankyo was even in the picture.
The figure below presents a colour-coded snapshot of the different stages in Ranbaxy's life, and who were at the helm during each of them.
The thoughts and opinions shared here are of the author.
Check out our end of season subscription discounts with a Moneycontrol pro subscription absolutely free. Use code EOSO2021. Click here for details.