“In 1989, GV Prasad [current co-chairman and MD of Dr Reddy’s] joined the company while Satish Reddy [chairman of Dr Reddy’s] joined in 1992," says BPS Reddy. “That’s when I thought, ‘Why can’t I establish a laboratory where I can implement my own philosophy, give jobs and opportunities to people who came from villages, like me?’ Because the freedom will come down with the next generation joining the management."
The rest is history. With ₹90 lakh from his savings, and by selling a plot of his land and his shares at Dr Reddy’s, BPS Reddy set up a laboratory, Hetero, in Hyderabad in 1993. Today, the company boasts over 21,000 employees and has grown into one of India’s best-known pharma companies, with businesses across active pharmaceutical ingredients (API), generics and biosimilars. It is the largest maker of antiretroviral drugs used for HIV patients and makes one out of every two Remdesivirs—it has seen soaring demand as Covid-19 cases peaked—across the globe.
“We always want to stay relevant," says Bandi, managing director, Hetero Group of Companies. “As a business, we had expanded to generics and biotech over the past few years. Last year, we stumbled across vaccines." The decision to foray into the lucrative vaccine business came on the back of a deal between Dr Reddy’s and the Russian Direct Investment Fund (RDIF), the sovereign wealth fund of the Russian government responsible for selling the Sputnik V vaccine. Sputnik V is the world’s first Covid-19 vaccine—registered as early as August 2020—that Dr Reddy’s wanted to bring to India.
![]() “What happened with Sputnik V was that Dr Reddy’s signed the marketing licence," says Bandi. “Then we looked at the capability. Fundamentally, in any pandemic, you will not be successful if you are not self-reliant. And we felt that for Sputnik V to be successful in India, it has to be a made-in-India product. That"s how the conversation started with the Russians."
“What happened with Sputnik V was that Dr Reddy’s signed the marketing licence," says Bandi. “Then we looked at the capability. Fundamentally, in any pandemic, you will not be successful if you are not self-reliant. And we felt that for Sputnik V to be successful in India, it has to be a made-in-India product. That"s how the conversation started with the Russians."
Sputnik V is based on the human adenovirus, a common cold virus, which is fused with the spike protein of Sars-CoV-2 to stimulate an immune response, and was developed by Gamaleya National Research Institute of Epidemiology and Microbiology, with support from RDIF.
By November 2020, Hetero became the first maker of Sputnik V in India, and committed to make 100 million doses of it to the RDIF. “In September, we were not sure whether Sputnik V will be launched in India," recalls Bandi. “But someone has to take a risk. We felt we should take the risk."
![]() Since then, RDIF has tied up with four other manufacturers in India to make over 850 million doses of Sputnik V, making India the biggest global producer of the vaccine. Apart from Hetero, RDIF has also reached agreements with Bengaluru-based Stelis Biopharma, Gland Pharma, Panacea Biotec and Virchow Biotech.
Since then, RDIF has tied up with four other manufacturers in India to make over 850 million doses of Sputnik V, making India the biggest global producer of the vaccine. Apart from Hetero, RDIF has also reached agreements with Bengaluru-based Stelis Biopharma, Gland Pharma, Panacea Biotec and Virchow Biotech.
On May 17, Raichur-based Shilpa Medicare struck a separate deal with Dr Reddy"s to manufacture 50 million doses of the vaccine, taking the total production of Sputnik V in India to over 900 million doses. “RDIF has five partners, and Dr Reddy’s has also partnered with Shilpa Medicare," says a spokesperson for Dr Reddy’s.
Sputnik V boasts an efficacy of 91.6 percent, making it one of the world’s three vaccines with an efficacy of more than 90 percent. The other two are the Pfizer-BioNTech vaccine and Moderna"s mRNA-1273 vaccine.
“Making 100 or 200 million doses is a function of capital," explains Bandi. “Our focus is to quickly launch the product in India capability and capacity building will follow. We have seen that in the case of Remdesivir. Sputnik V definitely has the potential to be a game changer… I think it"s a reasonably good product and the Russians have done a good job with it."
![]()
GV Prasad, managing director of Dr Reddy"s Laboratories, entered into an exclusive distribution partnership with RDIF after helping to conduct the clinical trials of Sputnik required by law
Image: Harsha Vadlamani
The Sputnik V Saga
RDIF’s plan to manufacture a significant chunk of Sputnik V in India comes at a time when the country is going through one of the worst humanitarian crises in recent history. India is the world’s second-worst Covid-19-hit country, with over 25 million cases and more than 260,000 deaths as on May 17. From over 26,000 daily cases on March 15, the number has hovered around 400,000 cases a day in May, the world"s highest. Many of the states are under lockdowns, in attempts to curtail the cases.
“Sputnik V is definitely a part of the solution [to India’s crisis]," says Kirill Dmitriev, CEO of RDIF. “It is one of only three vaccines in the world with confirmed efficacy of over 90 percent, and its real-world use around the globe demonstrates that it has an excellent safety profile when compared to other vaccines. We’ve advocated vaccine cooperation from the very beginning of the pandemic. Obviously, no single vaccine can fully meet the demand of a country as large and diverse as India. So securing access to a portfolio of vaccines is the necessary approach."
![]() On May 6, RDIF had said that a single dose of Sputnik V can provide immunity of nearly 80 percent, significantly higher than the Covishield vaccine (70 percent) being administered in India. “The Sputnik Light vaccine significantly reduces the possibility of severe cases leading to hospitalisation, and only one injection is needed," says Dmitriev. “The single-dose regimen solves the challenge of immunising large groups in a shorter time—which is important during the acute phase of the spread of Covid-19—achieving herd immunity faster."
On May 6, RDIF had said that a single dose of Sputnik V can provide immunity of nearly 80 percent, significantly higher than the Covishield vaccine (70 percent) being administered in India. “The Sputnik Light vaccine significantly reduces the possibility of severe cases leading to hospitalisation, and only one injection is needed," says Dmitriev. “The single-dose regimen solves the challenge of immunising large groups in a shorter time—which is important during the acute phase of the spread of Covid-19—achieving herd immunity faster."
That could be relevant for a country like India where active cases haven’t shown any signs of slowing down. Add to it a laggard vaccination programme, and a single-dose vaccine holds significance. “Sputnik Light will be the game changer," repeats Bandi. “The difference is on the clinical side, not on the product side. Initially, they went after a 90 percent efficacy rate, and realised that with 70 percent efficacies, the AstraZeneca vaccine or Sinopharm are doing an equally good job. That’s when they realised they don"t need a 90 percent efficacy scenario for this pandemic."
Hetero is now set to start phase 3 trials for the vaccine after an expert committee appointed by the government asked it to study and monitor the vaccine"s immunogenicity on days 21, 24 and 48 of the trials. Conducting trials and providing the government with adequate data assumes significance since the vaccine is yet to be approved by some global health regulators, although 64 counties have given their nod.
Since its launch in August 2020, Sputnik V has been in the eye of a storm for multiple reasons. Despite the Russians claiming high efficacy levels, the vaccine was met with suspicion globally due to the lack of publicly available scientific data and bypassing processes. It was only in February that a peer-reviewed study in The Lancet confirmed its 91.6 percent efficacy.
In India too, Sputnik V didn’t have a smooth sailing. There was a long wait for its approval, despite the rising cases in the country. While Dr Reddy’s had struck a deal with RDIF to sell Sputnik V in India as early as August 2020, the vaccine was approved for use this April. The first batch of the vaccine arrived in India on May 1 and a regulatory clearance for the batch was given by the Central Drugs Laboratory in Kasauli on May 13. Dr Reddy’s did a soft launch of the vaccine on May 14.
![]()
“All the players had tied up with somebody or the other, so it was our turn to tie up with the Russian Gamaleya Research Institute and RDIF," GV Prasad had told Forbes India last September. Named after the world’s first satellite launched into orbit in 1957 during the Cold War, the vaccine has faced allegations of being a tool in Russia’s soft power during the pandemic. Even now, the World Health Organization (WHO), the European Medicines Agency (EMA) and the United States Food and Drug Administration (USFDA) are wary about approving the vaccine.
Over the next 12 months, Dr Reddy’s plans to vaccinate 125 million people, under a two-dose regimen. “Our goal is to steadily ramp up to hit 20-25 million doses per month," the company spokesperson says. "The requirements of our cold chain infrastructure [-18 0C] means we will begin with tier 1 cities and scale up to reach the rest of India." In India, Dr Reddy’s will be responsible for the regulatory, safety and pharmaco-vigilance aspects of the vaccine.
“There is significant and understandable scepticism about Sputnik V, both inside Russia and globally," says Judyth L Twigg, a professor of political science at the Virginia Commonwealth University and senior associate at the Global Health Policy Center of the Washington-based Center for Strategic and International Studies. “The doubts date back to Russia"s premature regulatory approval of Sputnik V in August 2020 before it had entered large-scale clinical trials. RDIF claims that this scepticism is politically motivated, but the underlying data to support its claims of perfect safety and high efficacy have still not been provided. RDIF also engages in aggressive criticism, bordering on misinformation, about other Covid-19 vaccines, inviting accusations that it is, in fact, RDIF that is politicising the situation."
![]() But that hasn’t stopped Indian manufacturers who have struck deals with RDIF over the past few months. “Sputnik V has one of the highest effectiveness among all the vaccines," says Anand Khedkar, head of R&D at Stelis Biopharma, an arm of Bengaluru-based Strides Pharma, which is making 200 million doses of Sputnik V. “It will certainly play a significant role in the vaccination drive in India. RDIF was looking to manufacture significant volumes of Sputnik V. As part of its drive to identify the manufacturing partner, it got in touch with Stelis. Based on the expertise available at Stelis, we decided to partner with RDIF."
But that hasn’t stopped Indian manufacturers who have struck deals with RDIF over the past few months. “Sputnik V has one of the highest effectiveness among all the vaccines," says Anand Khedkar, head of R&D at Stelis Biopharma, an arm of Bengaluru-based Strides Pharma, which is making 200 million doses of Sputnik V. “It will certainly play a significant role in the vaccination drive in India. RDIF was looking to manufacture significant volumes of Sputnik V. As part of its drive to identify the manufacturing partner, it got in touch with Stelis. Based on the expertise available at Stelis, we decided to partner with RDIF."
In India, Dr Reddy’s has an exclusive distribution partnership with RDIF after it helped conduct the clinical trials required by law. “The April 12 registration in India for Sputnik V was made possible by our joint work," says Dmitriev.
India’s Sputnik Makers
“India is RDIF’s crucial production partner," adds Dmitriev. “India is slated to become the biggest global producer of Sputnik V by far. Production in the country could reach 50 million doses per month by the summer, with a possible scale-up." However, part of the reason why RDIF was keen on India was because the vaccine maker has been struggling to make the vaccine within Russia. Only recently, RDIF signed three deals with Chinese manufacturers to make 260 million doses of the vaccine, a fraction of what it intends to make in India.
“Given India"s situation, any safe, effective vaccine that can be made quickly and in significant quantity will be important," adds Twigg. “To date, Russia has struggled to produce enough Sputnik V domestically to meet global commitments. Making Sputnik V in India for use in India could be an important part of India"s plan. It appears that production in Russia of the Sputnik V first dose has been more successful than the second, and Sputnik Light is just the first dose. In principle, it should be possible for India to scale up Sputnik Light production relatively quickly."
All the Indian manufacturers will have to conduct bridging trials in order to show bio-equivalence before getting approval to sell their vaccines. They have, however, begun production of the vaccine. “The timelines depend on the regulatory approval process," says Khedkar from Stelis Biopharma. “We will be ready to start supplying from September onwards."
![]()
A manufacturing facility of Hetero Biopharma in Jadcherla, Hyderabad
“We do not anticipate any manufacturing delays due to regulatory requirements to conduct comparability studies for the vaccine coming from different manufacturers," says Dmitriev. “This is standard procedure."
Hetero has started making the vaccine and is looking at making it available as early as July. “Initially our understanding was that there will be ready technology and it will be easy to transfer, and we can start making it," says Bandi. “Unfortunately, because of the speed at which Sputnik V was developed, particularly the idea of getting the product to market… even RDIF and the Russians have not clearly invested time and effort in the building and scaling up of the product because no one had imagined that we"ll be in a scenario where we will need 2 billion doses."
Hetero had to invest its own “resources, time, energy, capital and people" over the past months to set up the technology platform needed to scale up capacity. “We felt we had to do our own bottom-up approach," says Bandi. “So, we invested and did not take any investment from anyone."
RDIF remains tight-lipped on how many of the 852 million doses it manufactures in India will be sold within the country. “We do not have visibility of that," says Khedkar. “We will manufacture and supply to RDIF. RDIF will then distribute to various countries, including India." Apart from India, RDIF has production agreements with roughly 20 production facilities in 10 countries.
With Sputnik Light in the mix, RDIF will be able to double its target of vaccinating 800 million people to 1.6 billion people within a year, as and when approvals for the single-dose vaccine come through. Bahrain has approved Sputnik Light for use. “We are in constant communication with governments and local regulators as we expect a number of new registrations in the coming days and weeks," says Dmitriev. “WHO and EMA inspectors were in Russia recently and getting their approval remains one of our priorities." In India, Dr Reddy’s has confirmed that it is in discussions with the government to get approvals for Sputnik Light.
Why does India need Sputnik v?
“Everyone knows that there is a demand-supply gap," says Bandi. “So it is a wide-open game for everyone. In a country like India, we need about five to six vaccines at full capacity to handle the current situation."
The first batch of Sputnik V containing 1.5 lakh doses—imported from Russia by Dr Reddy’s—arrived in India on the day the country opened vaccinations for all adults aged above 18, roughly 60 percent of the country’s 1.34 billion population. A second consignment carrying 60,000 doses arrived on May 16. The second consignment will be of the same quantity. “It is hard to say at the moment how much of India’s supply constraints Sputnik V can alleviate," says Satyajit Rath, a scientist at the National Institute of Immunology and the adjunct professor at the Indian Institute of Science Education and Research. “The Indian manufacturers have so far not given any indication of how many doses they will supply, and by when."
On January 16, India began what was touted as the world’s largest vaccination programme. As of May 17, India has vaccinated 183 million people using two of the publicly approved vaccines, Covishield and Covaxin. Of these, 40 million people have received both doses, while 143 million have received the first dose. But the programme has run into a quagmire due to a serious shortage of vaccines. While India’s daily vaccination touched over 4 million doses a day in April, the number has since fallen sharply to less than 700,000 doses in May.
India had initially planned to vaccinate 300 million people, including health care and frontline workers, by July, before vaccinating everyone else. On April 18, the central government devolved inoculation responsibility to the states and the private sector, departing from its original plan. Since then, numerous states such as Rajasthan, Madhya Pradesh, Uttarakhand, Tamil Nadu, Maharashtra, Karnataka, Andhra Pradesh, Telangana, Haryana and Delhi decided to float tenders, inviting global participation in procuring vaccines.
So, the likes of Hetero, which is looking to ramp up manufacturing, has a massive opportunity to inoculate a significant population in India. Until now, about 90 percent of the country’s vaccine needs were being met by Covishield, and the rest by Covaxin. The Serum Institute of India (SII), maker of Covishield, makes between 60 and 70 million doses a month, and is looking to ramp up production to 100 million doses monthly. On the other hand, Covaxin manufacturer Bharat Biotech has laid out plans to produce 700 million doses annually, up from the 5 million-odd doses it currently produces a month.
“Obviously, it is likely to take many months before the fully vaccinated make up the majority of the population… yet, that is what is probably needed for vaccination per se to have a major effect on future Covid-19 outbreaks," explains Rath.
At a time when more Indians are willing to get vaccinated, any added capacity to vaccination is welcome. However, without ramping up the number of doses being made available, 1.5-odd lakh doses of Sputnik V will only remain symbolic, says Anant Bhan, a global health, bioethics and health policy researcher. “Sputnik V will certainly add to the kitty, but vaccine imports are unlikely to make a significant dent, given the large population that needs to be vaccinated," he adds. “This is why it is more important to watch out for manufacturers of the vaccine in India who are making it available locally. By when the vaccine will be made available and at what quantities and price points are also crucial."
Sputnik V is priced at ₹995 per dose, including taxes. “At this stage, all vaccines are important and are our best bet in the fight against Covid-19," says the Dr Reddy’s spokesperson. “It is important that as many people as possible get vaccinated in the shortest possible time. Sputnik V is a safe and efficacious vaccine and an important element in the overall vaccination drive for the country."
In contrast, SII had announced that it would sell Covishield for ₹300 per dose to states and at ₹600 per dose to private hospitals. Bharat Biotech has pegged prices at ₹400 per dose for states and ₹1,200 for private hospitals. Both the companies were selling to the central government at ₹150 a dose.
“More than 850-million doses from Sputnik V overall seems like a decent number, but whatever is being made available in India needs to be ramped up because of the population requirements," says Bhan. According to him, states that are procuring the vaccine directly should aim to administer it for free, so that there are no inequities in access.
That said, concerns around the effectiveness of both Sputnik V and Sputnik Light continue to remain a cause of worry. “The reported 80 percent effectiveness of Sputnik Light is almost certainly overstated, based on information that has been released on the methodology for arriving at that result," says Twigg, adding that data on the efficacy of the vaccine against variants is unreliable. “However, it does appear that Sputnik V is a safe and effective vaccine, even if not at the levels the RDIF is advertising, and therefore could play an important role in helping India out of its current situation."
Bhan agrees, pointing out that a few global regulators flagging concerns around Sputnik is all the more reason to track the vaccine closely despite its emergency use authorisation in India. There is another quality metric that Sputnik V fails to meet at this time, he explains, which is that of WHO’s Strategic Advisory Group of Experts on Immunization that has given approvals for emergency use of many vaccines, including those by Pfizer, Moderna, AstraZeneca and Sinopharm from China.
“They have still not approved Sputnik V. From what I understand, the reason is they were not satisfied with the data, but we have to see what is officially said by WHO," he says. “Of course we need more vaccines in India, but we also have to be assured that India’s regulator has looked at the data in adequate detail, and has checks and balances in place even when Sputnik V starts being administered so that you can intensively follow-up on any cues related to safety and efficacy that might be relevant."
On May 12, in a letter to The Lancet, titled ‘Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial’, an international group of scientists had said that notwithstanding previous issues related to lack of transparency [in previously published phase 1/2 trials], the interim results from phase 3 results of Sputnik V also raise concerns. “Access to data underpinning study findings is imperative to check and confirm the findings claimed. It is even more serious if there are apparent errors and numerical inconsistencies in the statistics and results presented. Regrettably, this seems to be what is happening in the case of the Sputnik V phase 3 trial," the letter says. “We have a serious concern regarding the availability of the data from which the investigators draw their conclusions."
In response, the Gamalaya Research Institute has disputed these claims, stating that the reporting of the interim analysis in the phase 3 Sputnik V clinical trial fully complies with regulatory standards. "It is on this basis that Sputnik V has received registration in 51 countries, which confirms our full transparency and compliance with regulatory requirements." It also states that the "safety and immunogenicity of the Sputnik V vaccine has been confirmed in multiple studies".
For now, notwithstanding concerns over data, joint clinical trials of Sputnik V and AstraZeneca, the world’s first trials of a Covid-19 vaccine combination, are under way in Azerbaijan. “The study will evaluate the immunogenicity and safety of the combined use of the two vaccines," says Dmitriev. “Regulatory approval to conduct clinical trials were also obtained in the UAE last week and we expect approval in Belarus soon."
All this could mean another potential vaccine. For the likes of Hetero and Stelis, it means bigger opportunities as Indian pharmaceutical companies look to ramp up their vaccine play. “There will be a problem today and one tomorrow," says Bandi. “For now we have put our bet on Sputnik and we will bring value to the table and find a solution for the pandemic. What we feel is that, there will be newer technologies such as mRNA that are here to stay. I don’t believe we will be entirely dependent on foreign companies eventually and we will have an opportunity to play there."
With Sputnik V now ready to join Covishield and Covaxin in India’s vaccination programme, Bandi and the others certainly will have their hands full. It will take a concerted effort to fix India’s troubles.

 Vamsi Krishna Bandi (left), managing director of Hetero Group of Companies, and his father Bandi Parthasaradhi Reddy, founder and chairman of Hetero Group of Companies
Vamsi Krishna Bandi (left), managing director of Hetero Group of Companies, and his father Bandi Parthasaradhi Reddy, founder and chairman of Hetero Group of Companies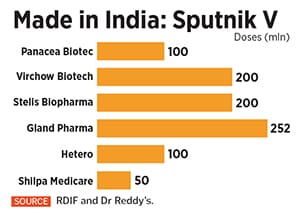 “What happened with Sputnik V was that Dr Reddy’s signed the marketing licence," says Bandi. “Then we looked at the capability. Fundamentally, in any pandemic, you will not be successful if you are not self-reliant. And we felt that for Sputnik V to be successful in India, it has to be a made-in-India product. That"s how the conversation started with the Russians."
“What happened with Sputnik V was that Dr Reddy’s signed the marketing licence," says Bandi. “Then we looked at the capability. Fundamentally, in any pandemic, you will not be successful if you are not self-reliant. And we felt that for Sputnik V to be successful in India, it has to be a made-in-India product. That"s how the conversation started with the Russians."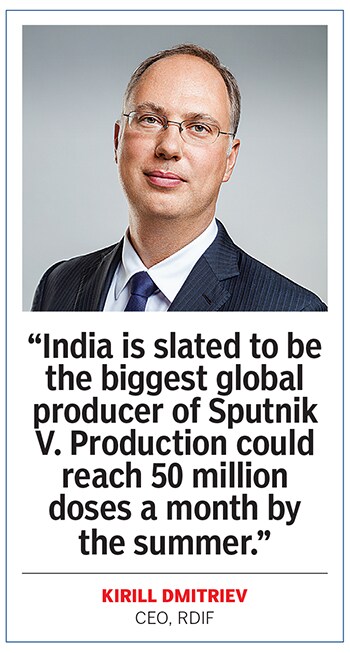 Since then, RDIF has tied up with four other manufacturers in India to make over 850 million doses of Sputnik V, making India the biggest global producer of the vaccine. Apart from Hetero, RDIF has also reached agreements with Bengaluru-based Stelis Biopharma, Gland Pharma, Panacea Biotec and Virchow Biotech.
Since then, RDIF has tied up with four other manufacturers in India to make over 850 million doses of Sputnik V, making India the biggest global producer of the vaccine. Apart from Hetero, RDIF has also reached agreements with Bengaluru-based Stelis Biopharma, Gland Pharma, Panacea Biotec and Virchow Biotech.
 On May 6, RDIF had said that a single dose of Sputnik V can provide immunity of nearly 80 percent, significantly higher than the Covishield vaccine (70 percent) being administered in India. “The Sputnik Light vaccine significantly reduces the possibility of severe cases leading to hospitalisation, and only one injection is needed," says Dmitriev. “The single-dose regimen solves the challenge of immunising large groups in a shorter time—which is important during the acute phase of the spread of Covid-19—achieving herd immunity faster."
On May 6, RDIF had said that a single dose of Sputnik V can provide immunity of nearly 80 percent, significantly higher than the Covishield vaccine (70 percent) being administered in India. “The Sputnik Light vaccine significantly reduces the possibility of severe cases leading to hospitalisation, and only one injection is needed," says Dmitriev. “The single-dose regimen solves the challenge of immunising large groups in a shorter time—which is important during the acute phase of the spread of Covid-19—achieving herd immunity faster."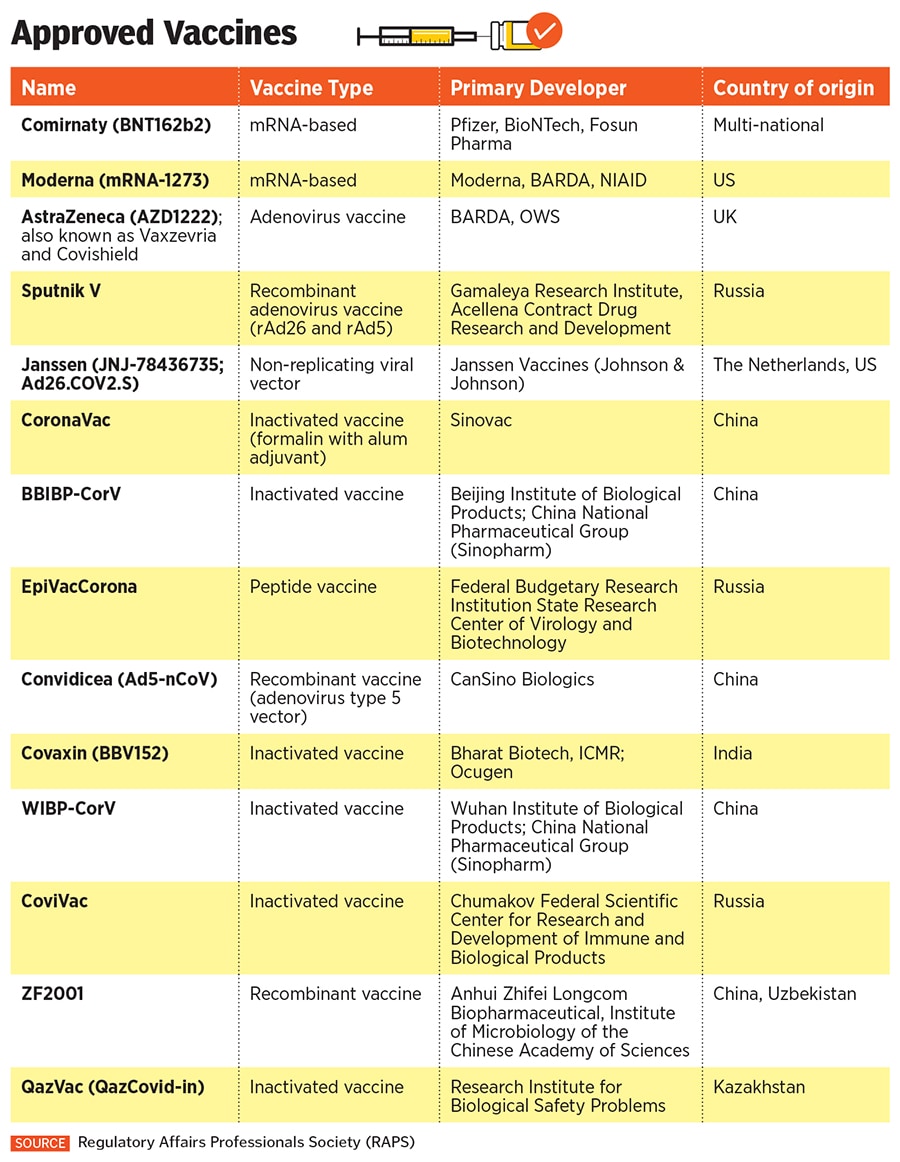
 But that hasn’t stopped Indian manufacturers who have struck deals with RDIF over the past few months. “Sputnik V has one of the highest effectiveness among all the vaccines," says Anand Khedkar, head of R&D at Stelis Biopharma, an arm of Bengaluru-based Strides Pharma, which is making 200 million doses of Sputnik V. “It will certainly play a significant role in the vaccination drive in India. RDIF was looking to manufacture significant volumes of Sputnik V. As part of its drive to identify the manufacturing partner, it got in touch with Stelis. Based on the expertise available at Stelis, we decided to partner with RDIF."
But that hasn’t stopped Indian manufacturers who have struck deals with RDIF over the past few months. “Sputnik V has one of the highest effectiveness among all the vaccines," says Anand Khedkar, head of R&D at Stelis Biopharma, an arm of Bengaluru-based Strides Pharma, which is making 200 million doses of Sputnik V. “It will certainly play a significant role in the vaccination drive in India. RDIF was looking to manufacture significant volumes of Sputnik V. As part of its drive to identify the manufacturing partner, it got in touch with Stelis. Based on the expertise available at Stelis, we decided to partner with RDIF."