Craig Venter mapped the human genome, now he's trying to cheat death
Craig Venter, the man who mapped the human genome, is back with a $25,000 physical he hopes can extend your life—and make him a billionaire


 Craig Venter’s DNA breakthrough was one of the great scientific accomplishments of the 20th century, yet he never won a Nobel PrizeImage: Ethan Pines for ForbesThe world’s most extreme physical exam starts in the world’s plushest exam room, complete with a couch, a private bathroom and a teeming fruit plate. It will be my home for an entire day. First come the blood tests, vial after vial. Then two 35-minute sessions in an MRI tube, where REM and U2 try to drown out the clanks as the machine takes pictures of my entire body. There’s an ultrasound of my heart. Salade Niçoise for lunch. A stool sample. A cognitive test in which letters flash on a computer screen at a dizzying pace. And a CT scan of my heart as well, which originally seemed so over-the-top for someone my age that I tried to get out of it.
Craig Venter’s DNA breakthrough was one of the great scientific accomplishments of the 20th century, yet he never won a Nobel PrizeImage: Ethan Pines for ForbesThe world’s most extreme physical exam starts in the world’s plushest exam room, complete with a couch, a private bathroom and a teeming fruit plate. It will be my home for an entire day. First come the blood tests, vial after vial. Then two 35-minute sessions in an MRI tube, where REM and U2 try to drown out the clanks as the machine takes pictures of my entire body. There’s an ultrasound of my heart. Salade Niçoise for lunch. A stool sample. A cognitive test in which letters flash on a computer screen at a dizzying pace. And a CT scan of my heart as well, which originally seemed so over-the-top for someone my age that I tried to get out of it.
“In Vietnam, I used to do autopsies on 18-to-22-year-olds, and a lot of them had cardiovascular disease,” J Craig Venter, the architect of the process, says with a shrug, before adding, ominously, “We find things. The question is what you do with it.”
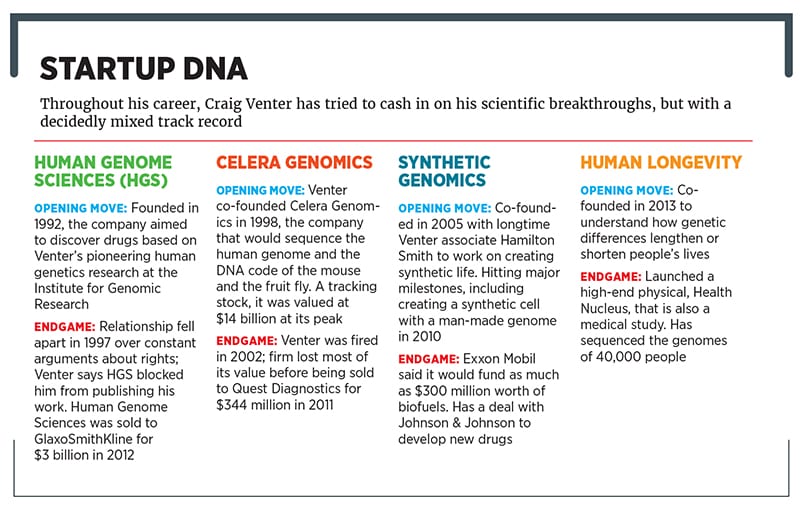
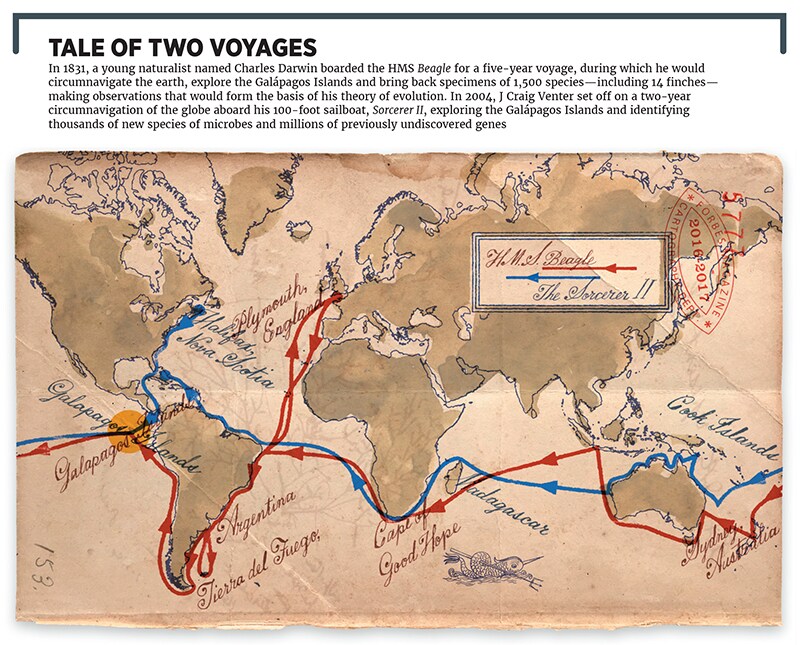
Yes, it’s that Craig Venter, the man in the late 1990s who, frustrated by the slow progress of the government-funded Human Genome Project, launched an effort that sequenced human DNA two years earlier than planned (he was subsequently the first human to have his complete DNA sequenced). He hasn’t slowed down since. He sailed around the world in a voyage inspired by Darwin’s journey on the Beagle, discovering thousands of new species along the way. He has created synthetic life and started three companies, and was almost a billionaire before being fired from one of the most promising, Celera Genomics.

Now he’s back with his most ambitious project since his historic breakthrough 17 years ago. He’s raised $300 million from investors, including Celgene and GE Ventures, for a new firm, Human Longevity, that’s trying to take the DNA information he helped unlock and figure out how to leverage it to cheat death for years, or even decades.
Core to the effort is the $25,000 executive physical, branded the Health Nucleus, that I’m taking (Disclosure: I got tested for free). It’s certainly very thorough—and, to many doctors, precisely the wrong approach, owing to all the false positives. “Study after study of various kinds of screening measures has shown they do more harm than good,” says Steven Nissen, the chairman of cardiology at the Cleveland Clinic. “You do a total body MRI and you’re lucky if you don’t find something. I don’t think it’s good medicine.”
Venter scoffs. “We’re screening healthy people, and a lot of physicians don’t like that,” he acknowledges. “My response is: How do you know they’re healthy? We use a definition of health out of the Middle Ages: If you look okay and you feel okay, you’re deemed healthy. We have a different way of looking at people.”
Now 70, Venter cites himself. Last year, he underwent his own physical and says he found prostate cancer, which was removed last November. The man he has called his “scientific muse”, Nobel laureate Hamilton Smith, 85, found he had a deadly lymphoma in his lung. It has also been treated, and Smith says his prognosis is good.
The famously gruff Venter is entirely comfortable ticking off the establishment, no matter what that establishment is, and the feeling is mutual. His DNA breakthrough was one of the great scientific accomplishments of the 20th century, yet he never won a Nobel Prize. Academics view him as someone interested in profits over science. “He’s a very insecure person who compensates by coming across as very arrogant and aggressive,” says one former collaborator. Similarly, Venter’s discoveries have upended industries, yet his business track record, including a brief flirtation with billionairehood, is chequered, as connections to past backers and bosses have gone up in flames. “He has irritated a lot of people,” says Harvard genetics professor George Church, a Venter fan. “It’s a pity.”
Thus, Human Longevity offers Venter a last chance to square his legacy, awe the scientists and make billions in the process, all the while shaking the foundation of a topic that precisely 100 percent of homo sapiens have a keen interest in: How and when each of us will die.
Venter has displayed potential, both achieved and unrealised, almost since birth. Growing up in Millbrae, California, near what was emerging as Silicon Valley, he had such bad grades that by high school, his worried mother sometimes checked his arms for track marks. The first glimmer of his future success was in swimming. He was initially mediocre, but when a coach sent him home for the summer with tips, his competitive streak kicked in. He spent three months training furiously and never again lost a race. “Had things been different, I would have been competing for the Olympics,” Venter says. “But Lyndon Johnson changed that for me with the draft.”
Swimming unlocked his potential, but Vietnam made him who he is. At the age of 20, he served as a Navy hospital corpsman, triaging troops who came back from battle, including the Tet Offensive. Deciding who would live and who would die was so traumatic that he says he considered suicide and swam far out to sea intending to drown. He says he had a change of heart a mile out after a shark prodded him. But he’d go through Vietnam again. “Knowing the outcome and what it did for my personal growth, I would force myself to do it again if I had the choice,” Venter says.After he returned to the States, he went to community college, then the University of California, San Diego, where he initially wanted to be a doctor but discovered science. He eventually completed his PhD in physiology and pharmacology, became a professor at the State University of New York at Buffalo in 1976 and, in 1984, joined the National Institutes of Health (NIH).
At the NIH the themes that would define his career locked into place: Productivity, perceived greed, the conflicts between pure science and industry money. Using a new technology, he discovered thousands of human genes. The NIH made the unprecedented decision to patent them in his name, and colleagues blamed Venter, calling him greedy. Nobel laureate James Watson said he was “horrified”. Venter insists he was always against the patents but that the NIH did it anyway.
Frustrated, he started a non-profit institute in 1992, with a unique model. He raised money from venture capitalists, on the condition that he share his data with a for-profit company, Human Genome Sciences, before he published it. The relationship ended unhappily in 1997 because of arguments over data disclosure, with Venter walking away from $40 million in research funding. “I paid a lot of money to get rid of [Human Genome Sciences],” Venter says.
But in 1995, Venter’s institute made a real breakthrough: The first genome, or map of the genetic code of an organism, in this case a type of bacterium. It was a suggestion from Ham Smith. They had met at a scientific conference in Spain in 1993 and gone out drinking, starting a two-decade-plus collaboration.
Foreshadowing his later race with the Human Genome Project, Venter and Smith’s bacterial genome map beat similar projects in academia by many months.
That led a California unit of lab equipment maker PerkinElmer, which made DNA sequencers, to approach Venter. If he could sequence a bacterial genome, why not use the company’s newest machines to sequence a human genome?
Venter couldn’t say no, which led to Celera Genomics’s founding in 1998. It not only succeeded in overtaking the $3 billion Human Genome Project, an international consortium funded largely by the US government, but it also mapped the genomes of the fruit fly and the mouse, both important laboratory animals. In the process, Venter angered scientists globally, aghast that such research would be driven by profit rather than knowledge. At the time, James Watson reportedly became so enraged that he compared Venter to Hitler, asking colleagues who they were going to be—Chamberlain or Churchill?
But the pressure of private enterprise ultimately spurred results, both at Celera and the public group, which improved their methods and accelerated their research. As a result, the two groups jointly announced they had mapped the entire human genome—an achievement that our grandkids will be reading about in their textbooks—at the White House on June 26, 2000.
In the age of the dotcom boom, Celera became a highflier, raising $855 million in a stock offering in February 2000 and peaking at a market capitalisation of $14 billion just before the entire market started to collapse in March.
Venter’s stake briefly surpassed $700 million. He says he gave half his shares to his non-profit foundation, which then sold half of them, netting more than $150 million, which has funded his science ever since.
It was a necessary scientific nest egg. Celera struggled to invent drugs and diagnostic tests based on its pioneering research, and Venter bickered constantly with the board. They wanted Celera to become a pharma giant and invent medicines in-house. Venter simply wanted to be a scientist and sell other companies his data. He was fired in January 2002, days before a quarter of his stock options would vest. “Being fired in the way it was done was about as slimy as anybody could do it,” Venter says. Celera limped along until 2011, when it was sold to Quest Diagnostics for $344 million. (Forbes estimates that Venter’s current net worth, based on his stakes in his two startups, is $300 million.) Venter’s baby had essentially been sold for parts.
With Human Longevity, Venter hopes to solve the problem that ultimately limited the efficacy of Celera and the Human Genome Project. Those two groups produced an “average” DNA sequence. That’s incredibly important for a science textbook, but for individuals, it’s the differences—how one person’s genes are different from another’s, leading to different noses, eye colours and, yes, diseases—that matter.
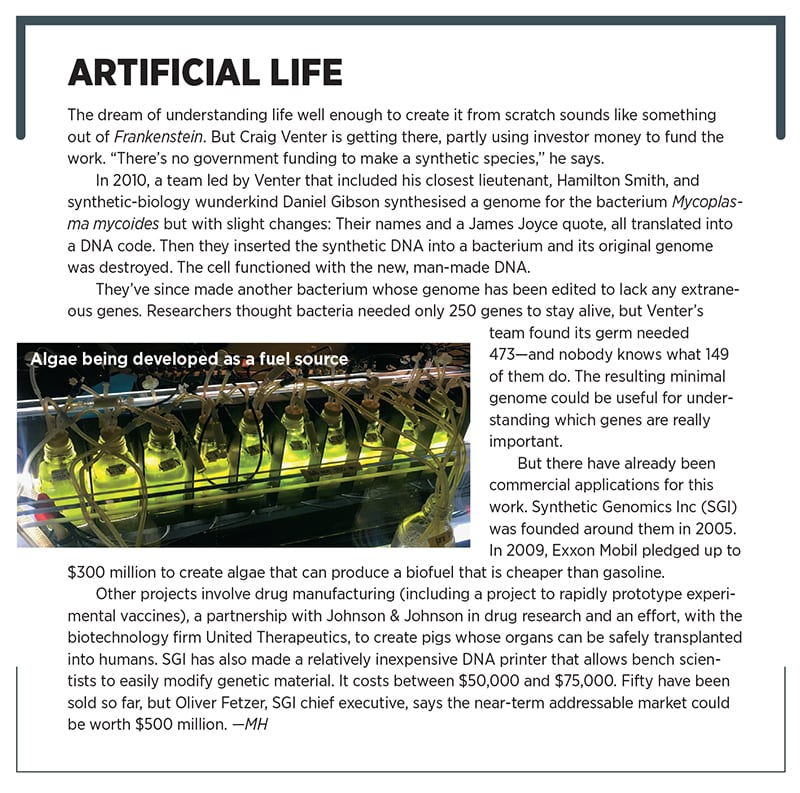
Venter says that, thanks to new technology, he can generate the data that can determine those differences. At Celera, Venter loved to show off his 25,000-square-foot rooms of DNA sequencing machines. But just one modern desktop DNA sequencer is as powerful as a thousand of those rooms and can map a person’s genome in days for about $1,000. The original Human Genome Project took more than a decade and at least $500 million to do the same thing. (Illumina, the San Diego firm that makes the desktop sequencers, is a big investor in Human Longevity.)
Human Longevity initially sequenced DNA from 40,000 people who had participated in clinical trials for the pharmaceutical companies Roche and AstraZeneca. Venter says this work has led to the discovery of genetic variations that can be found in young people but not older ones—meaning the young folks had genes incompatible with surviving into old age.Figuring out what these genes do could do could be the kind of breakthrough that would turn the promise of genome sequencing into a lifesaver.
Venter decided that he also needed a study of people that could collect even more data than you can get from a clinical trial. Hence, the $25,000 physical. And because people pay, it’s not only a source of data but also a revenue generator. At the moment, close to 500 people have gone through the physical. Venter hopes to be able to serve 2,000 annually as early as this year, which would generate $50 million in revenue. This isn’t exactly covered by Medicare. The market, for the moment, will be the wealthy and the occasional company looking out for key executives—the promise of health as the ultimate luxury item.
Doctors hate it. “I’m massively sceptical,” says Benjamin Davies, a urologist at the University of Pittsburgh. “We’ve been down this road of investigating healthy patients, and it’s been a sordid road.” He points to a recent study that used CT scans to screen for lung cancer: 60 percent of patients needed follow-up tests, but only 1.5 percent had cancer. Otis Brawley, the chief medical officer of the American Cancer Society, said Venter’s work sounded like “fascinating science”, so long as the people taking the physical understand that this is research, not medicine.
Venter believes the problem with earlier screening tests is that they give too little data, not too much. He is his own evidence. He was the first person to get his DNA sequenced, and the results made him think his risk for most types of cancer was low. When he got prostate cancer, he asked his researchers why. They found what he calls “the likely perpetrator”.
It’s a change in the way his body responds to the hormone testosterone. Testosterone works by tripping a cellular receptor (think of it as a switch). The gene for that receptor is more effective if it has fewer “repeats” (bits of repeated, garbled genetic code). Testosterone makes prostate cancer grow, so a man with 22 repeats and an inefficient receptor has a lowered risk of the disease. Venter’s androgen receptor had just six repeats.
“Basically, I have a supersensitive testosterone receptor,” Venter says. “Everybody thought I had balls of steel. In fact, I have only six repeats in my androgen receptor.”
But Venter’s constant search for more data about his own biology also made the problem worse, illustrating one of the true dangers of the $25,000 physical. Years before, Venter learnt that his testosterone levels were low and decided to take testosterone supplements. (most doctors don’t recommend doing this.) It almost certainly made his tumour grow faster.
About 40 percent of Health Nucleus’s patients have found out they have something serious. Some, like Ham Smith’s lung cancer, absolutely needed to be treated.
Venter insists Smith’s tumour might have killed him had it been discovered a few weeks later. But for most of Human Longevity’s patients, the results are not so clear-cut. I’m lucky: My MRI results showed nothing save that my hippocampus, a part of the brain that forms memories, is of only average size. (My DNA sequence isn’t in yet.)
I’ve been thinking a lot about what I would do if I’d learnt about a tumour or an aneurysm, and whether this whole endeavour is a bad idea. But I also haven’t been able to get myself to regret going through it. Knowledge about yourself is a very seductive offer. It’s one that Venter hopes will give him the data to finally deliver on the genome’s promise.
First Published: Mar 25, 2017, 06:41
Subscribe Now