Everything, that is, except proof that any of their vaccines work.
Unlike their Western competitors, the Chinese companies have not disclosed data from late-stage clinical trials that would show whether their vaccines are effective, and regulators in China have not officially approved them.
That has not deterred local governments across the country, which have begun an ambitious vaccination campaign. The goal is to inoculate 50 million people — roughly the population of Colombia — by the middle of February, before the Lunar New Year holiday, when hundreds of millions of people are expected to travel.
China, where the virus first emerged a year ago, is going to great — and scientifically unorthodox — lengths to prevent a resurgence of the outbreak. While Beijing has not officially announced the vaccine target, the government has signaled the rollout will be managed in much the same way as the outbreak, through a top-down approach that can mobilize thousands of workers to produce, ship and administer the shots. Local officials were told that the drive was a “political mission.”
The campaign will focus on what China calls “key priority groups,” including doctors, hotel employees, border inspection personnel, food storage and transportation workers, as well as travelers. Irene Zhang, a 24-year-old student, got a vaccine on Dec. 22 in the city of Hangzhou before going to Britain next month for graduate school.
“Because my situation is pretty urgent and all the students around me going abroad have taken it, I feel it’s relatively reliable,” Zhang said.
Even before this current campaign, more than 1 million people had lined up to get vaccinated, bewildering scientists who have warned that taking unproven vaccines poses potential health risks. Their efforts now, which are wider in scope, are being rolled out in a similarly ad hoc fashion.
In southern Guangdong province, 180,000 people — mostly workers involved in food storage and transportation, quarantine facilities and border inspection — had been inoculated as of Dec. 22. In the eastern province of Zhejiang, 281,800 people had been vaccinated. In Wuhan, where the outbreak was first detected, the government said it had designated 48 vaccination clinics for its emergency program, which started Thursday.
China, which is testing five vaccines in Phase 3 trials, has not provided any information from this last stage to show the efficacy of these vaccines. By contrast, the United States and Britain started inoculations after reviewing and approving such trial data.
Instead, Chinese officials have issued broad statements with few details, assuring the public that the vaccines are safe and effective. Three of the vaccines have been approved only for emergency use. Last month, Liu Jingzhen, the chairman of Sinopharm, a state-owned vaccine-maker that has two vaccines in late-stage trials, said that none of the roughly 1 million people vaccinated so far had any adverse reactions and that “only a few had mild symptoms.”
The data and approval are expected to come within weeks. While there have been promising signs, they have come with caveats.
The United Arab Emirates and Bahrain said this month that a vaccine made by Sinopharm was effective, although they offered few details on how the conclusions were reached. Turkey said a vaccine made by Sinovac, a private Beijing-based vaccine-maker, has an efficacy rate of 91.25%, a finding that was based on preliminary results from a small clinical trial. Officials in Brazil said the Sinovac vaccine had an efficacy rate over 50% but put off releasing detailed data.
The scale and speed of the vaccination drive are the outgrowth of a centralized public health infrastructure in an authoritarian system. During the crisis, China showed how it can mobilize thousands of workers to reach millions of people it tested 11 million people in Wuhan in 10 days.
Chinese vaccine-makers have worked to ramp up their production, both for the country’s own needs and global exports. The Chinese government has promised to produce 610 million doses by the end of the year and expects to make more than 1 billion doses next year.
“When they say 50 million, they probably will do it,” said Jennifer Huang Bouey, a senior policy researcher at the RAND Corp. and a public health researcher. “The question is how much it would cost and what is the effect.”
The all-out effort has taken months of preparation. Since June, hospitals in Guangdong province have started construction on vaccination clinics, equipped them with refrigerators and installed refrigerated storage systems.
Sinopharm conducted drills this month. In the trial run, workers loaded boxes with the vaccines and ice packs, while company official tracked the vaccines’ temperature in real time during shipment.
China has some advantages in its rollout. Unlike the Pfizer vaccine, those made by Sinopharm and Sinovac are based on traditional methods that use inactivated or weakened forms of the virus, making them easier to store and distribute.
But the pitfalls are plenty, as the U.S. experience has shown. In the United States, just over 2 million people have received a COVID-19 vaccine, far short of the 20 million goal the government had set for this month. Hospitals had to prepare the frozen shots and find employees to staff the clinics.
As China has geared up, local officials have been surveying the number of people in the “key priority groups.” They had to “ensure there were no omissions,” according to a government document from Xinchang County in Zhejiang province.
Just two months ago, it appeared that demand could outstrip supply. The eastern city of Yiwu had offered 500 doses, which were used within hours.
Zhang, the student, said she had initially been hesitant to get a vaccine because everyone around her had told her to “wait and see.” Still, she tried to sign up in Yiwu but failed to secure a spot.
Then, on Dec. 21, Zhang heard that Hangzhou was going to start its own vaccination drive. She took a high-speed train that night and signed a rental contract with her friend in the city since the local authorities required proof of residence. The next day, she paid $35 and got the shot from Sinovac.
At the hospital, four or five people were waiting to get the vaccine, according to Zhang. The process took one hour, which included registering, getting the shot and waiting for 30 minutes to see if there were any adverse reactions.
“Everything was very calm and orderly,” she said. Before she left, the doctor warned her: Don’t take a shower. Don’t stay up late. Don’t eat foods that could irritate your stomach.
The government has stressed that the vaccination drive is voluntary, and people will have to pay for the inoculations. Yanzhong Huang, a senior fellow for global health at the Council on Foreign Relations and an expert on health care in China, noted that the two-dose regimen could cost about $70, putting it out of reach for the rural poor.
China could also have problems trying to persuade people to take the vaccine. Scientists warn that the lack of transparency could set off fears about taking a new vaccine, especially in an industry that has a history of quality scandals.
Tao Lina, a vaccine expert and a former immunologist at the Shanghai Center for Disease Control and Prevention, said he knew of several health care workers who had declined the shots. “In the minds of doctors, they feel that any drug that has not passed Phase 3 trials is unreliable,” Tao said.
Tao, who got a Sinopharm vaccine on Monday, said he felt confident that the vaccines were safe and effective, echoing officials’ comments that there have been no reports of serious, adverse reactions. But he added that the companies could do better in their messaging.
“If you say that it’s safe, then you should show all kinds of evidence to show that it’s safe,” he said.
Hminem Zhang, a 27-year-old sales employee in an internet company, said he wanted to get a vaccine because he traveled for work and feared a run on the shots if there was a resurgence of the virus. But he worries about the Chinese-made ones because “not many people have received it,” he said.
“I want to wait another month or two for some official data to come out,” said Hminem Zhang, who is based in the southwestern municipality of Chongqing. “And then, if there is no news about any side effects, I will get a shot.”

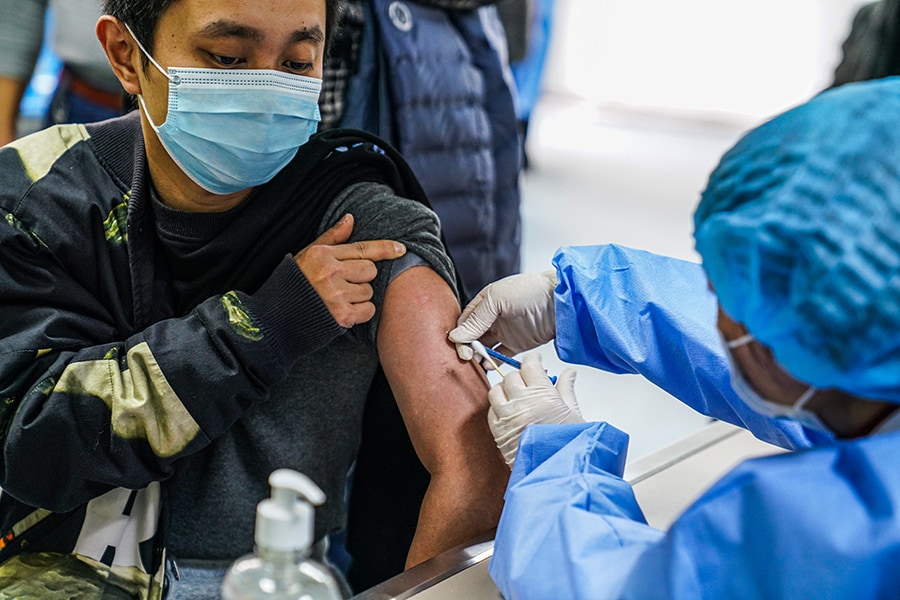 A local resident receives a Sinovac"s COVID-19 vaccine at a hospital on December 26, 2020 in Hefei, Anhui Province of China.
A local resident receives a Sinovac"s COVID-19 vaccine at a hospital on December 26, 2020 in Hefei, Anhui Province of China.