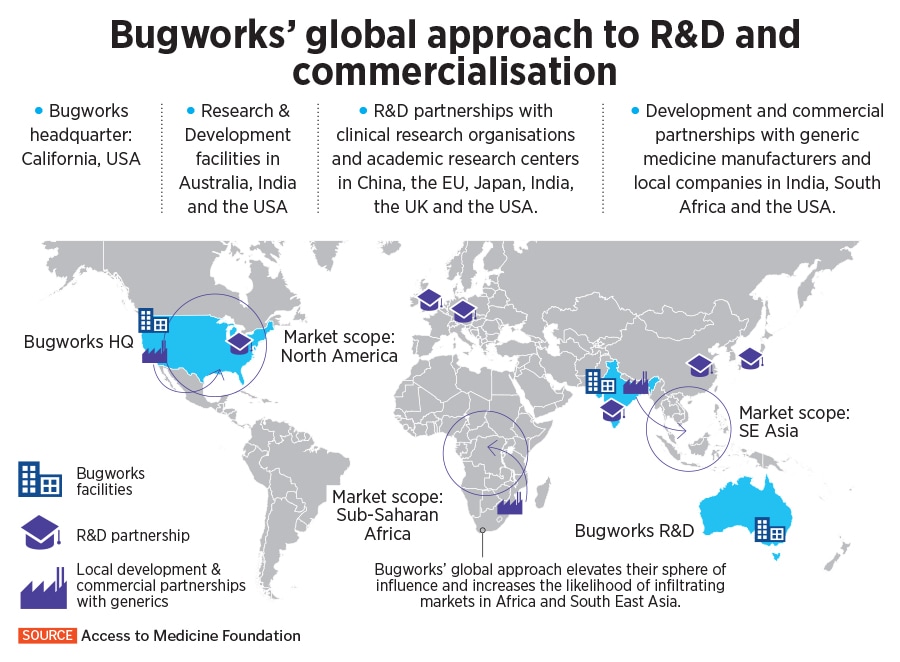
If they succeed, it will be the first time in more than 60 years that a new class of antibiotics will come to the market to combat ‘superbugs.’ And it will also be a significant milestone for drug discovery out of India.
Since November, Bugworks has been putting its first promising candidate, a molecule the founders have named BWC0977, through the first phase of clinical trials. The tests are being conducted in Australia. If all goes well, then, by around October this year, they will be ready to move into Phase 2 trials.
An eventual commercial drug, even if everything goes well and according to plan, is still at least 3-4 years away. But if it does come to the market, then Bugworks’ founders expect it to be able to combat bacteria that cause severe urinary tract infections, but also, eventually, pneumonia and infections of the bloodstream, kidney, stomach, skin and so on.
Anandkumar comes from a family “filled with doctors other than me", he says. He started his career in the semiconductor space, with a PhD in electrical and biomedical engineering and chip design, and he worked for some 15 years in that industry in the US, Europe, and Japan.
He moved to India in early 2000s and helped run a couple of startups. Most notably, he ran the Indian operations of Magma Design Automation, a fabless chip design company that was acquired by another semiconductor company Synopsys.
A personal health crisis turned his attention to life sciences in the early 2000s.
“I dealt with it by converting it into an opportunity and I realised that my life was meant to do something in health care," Anandkumar says.
With some friends in the Bay Area and in Bengaluru, he co-founded a company called Cellworks in 2007. That venture, which worked on simulations of cancer biology, grew into a provider of a platform used by leading hospitals in the US to customise treatment for cancer patients who are not responding to first-line treatment.
![]()
Anandkumar spun out Bugworks from Cellworks in 2013-14. Even back then, the writing was on the wall, he says. His exposure to science and tech around the world, and his own experience, had convinced him of that.
Today “we are coming out of Covid, but there is another one round the corner called AMR or antimicrobial resistance," he says.
This is emerged out of constant use of antibiotics around the world — and India is the biggest in terms of number pills popped, even bigger than China. “Bacteria have become much smarter and because we have abused antibiotics, we have exposed all out of weaponry to the enemy and the enemy has decided to adapt," Anandkumar says.
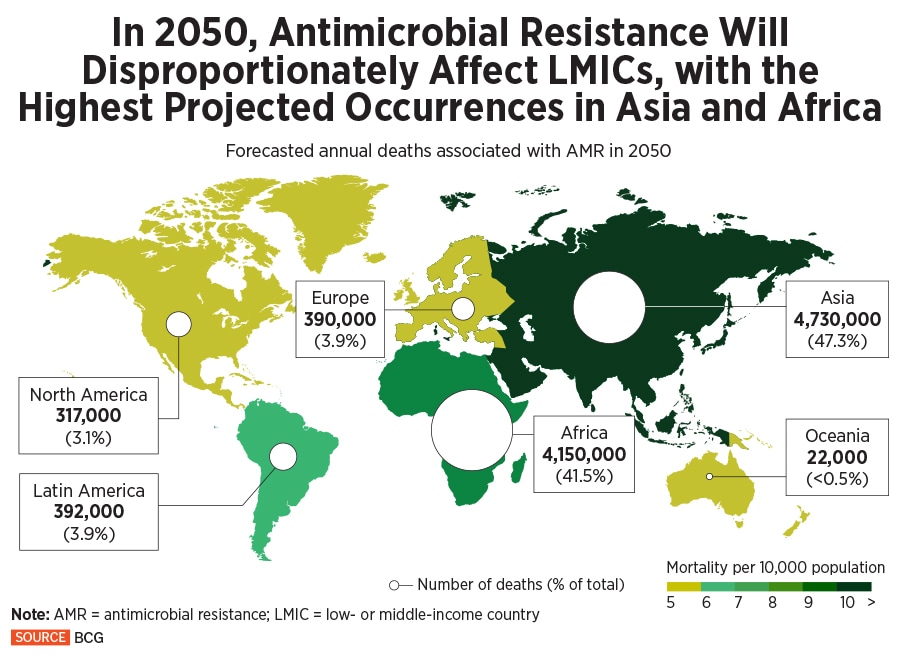
By 2050, low and middle-income countries will be disproportionately affected by AMR, Boston Consulting Group, a consultancy, notes in a February 2022 report. Cumulatively AMR deaths by then are projected to reach about 10 million people.
That apart, 30 percent of deaths in hospitals in India, when people visit for surgical procedures for instance, is due to hospital acquired infections of bacteria that are drug-resistant, Datta says. Another complication can be that bacteria can double every 20-30 minutes, which means that in a day, one bug can go to a billion and make the infection acute in a couple of days, he says. “So there is a huge unmet need for effective broad spectrum antibiotics."
The last time that a new antibiotic for the worst superbugs came out was in the 1960s, when medicines called ‘fluoroquinolones’ were introduced, Anandkumar says. And large pharma companies aren’t interested in antibiotics because there just isn’t enough money in them. In fact, AstraZeneca shut down its research and development centre in Bengaluru some six years ago, which at its peak had a strong team of 300 people, working on medicines for infectious diseases.
It’s much more lucrative to make drugs for cancer, diabetes and heart disease.
Datta, Hameed and Balasubramanian were all senior staffers at the AstraZeneca centre, collectively with half a century’s experience. The closure of that centre was, in a sense, Bugworks’s gain.
“Nobody"s going to fix problems in markets like India, Southeast Asia and Africa. So we are getting an opportunity to do it ourselves," Anandkumar says.
![]()
In terms of the science, Bugworks has found a way to attack bacteria in two places at once, hitting two enzymes needed for the bug to multiply and thrive—and the method has been found to be efficacious across different types of bacteria. Bugworks has tested its molecule on some 10,000 ‘bacterial isolates’ from around the world.
To describe Bugworks’s approach to fighting the bacteria, “we often use this phrase, it’s like hitting the ‘head and the heart’" Datta says. When two essential functions of the bacteria are attacked simultaneously it greatly reduces the probability that it can mutate and evolve to again become resistant to the antibiotic, he says.
The bacteria has multiple mechanisms to defend itself, Hameed says. One important way is something called an efflux pump that basically throws out the medicine that could have fought it. What Bugworks has done is that, instead of tackling the pump directly, they have found a way to make their drug invisible to it.
“Think of the efflux pump as bar bouncers," Datta says. And “Bugworks’s antibiotic, instead of looking like a trouble maker, seems innocuous to the bouncers."
“The concept of efflux pump inhibitors was there but not many had tried it," Hameed says. Overall, this combined strategy of targeting the bacteria at two places and also fooling the efflux pump mechanism has given Bugworks a new antibiotic, he says.
Key to phase 1 trials is establishing the safety of the drug. And Bugworks has struck international partnerships for this, including with Carb-X (Combating Antibiotic-Resistant Bacteria) and GARD-p (Global Antibiotic Research and Development Partnership). One important aspect of the safety tests in the case of this particular drug is to see if it causes any problems with the heart’s rhythm.
The drug is currently being developed for both intravenous and oral administration. If phase 1 goes through, then at phase 2, Bugworks has selected urinary tract infection as the first problem to tackle and tests will be done with around 200 people. Europe will be likely market for phase 2 because it has one of the best regulatory environments, Anandkumar says.
If all that works out, the phase 3 will involve testing it on about 2000 people.
“We ultimately want to launch this product in India. All of us in this company, the co-founders, are in our fifties, some in their sixties. We didn"t do this company to sell off to a big pharmaceutical company," he says.
If all goes according to plan, Phase 3 trials can start in 2024 and those trials will take another 12-18 months. At that stage, Bugworks’ founders are hoping to find partners from among both India’s large generics companies, and multinational drug companies to commercialise their product.
In the meantime, Bugworks has a strong combination of drug-discover knowhow and it has added on modelling and simulation in structural biology to create a platform and basket of molecules in antibiotics. Therefore, if BWC0977 fails, the venture may have other candidates in the pipeline.
The company has also been able to use the same platform, named GYROX, and apply it to cancer research. First, if they can sell their solutions and knowhow in this area, it adds revenue that can be ploughed into the drug research. Second, the best cancer drugs world over are very expensive for most people. Waiting for generics will be a decadal wait and that will continue to keep India behind by many years.
If companies like Bugworks can actually innovate in targeted areas of cancer drugs, then that could make an important difference for the entire biotech industry locally.
Support from the Indian government hasn’t been lacking, at least in the early stage. Today, there are several government-backed incubators and accelerators that offer basic research infrastructure and some financial aid as well, including grants and early-stage equity funding.
What is needed, however, is to go beyond the current stage of supporting, say 500 startups with 50 lakh rupees each or a crore each, to picking a small number of innovators tackling the top five diseases in India and “fund them deep" to the tune of Rs50-200 crore, according to Anandkumar.
In one of the advanced economies, it takes at least $500 million to bring a novel drug to the market. In India it might take $150 million. That funding just doesn’t exist for a small India startup today. This means more India billionaires like Biocon Founder Kiran Mazumdar-Shaw—who gave some early funding to Bugworks—and Infosys Co-founder Kris Gopalakrishnan will have to back life sciences startups, he says.
In addition to grants, Bugworks has currently raised about $20 million in equity funding, which will keep it going for another two years. So far, the biggest investors have been the likes of University of Tokyo Edge Capital UTEC — one of the top four funds in Japan, Global Brain, another Japanese firm, and Lightrock Ventures, which is a fully owned subsidiary of Lichtenstein Global Trust.
“Antimicrobial resistance (AMR) is fast becoming one of the most important global public health crises," Tejasvi Ravi, who leads health care investments at Lightrock, said in a statement in February, when the firm led Bugworks’ series B1 investment.
“Their in-house platform coupled with a truly global execution network, puts Bugworks in a unique position to deliver path-breaking solutions to combat AMR and cancer," Ravi said.
Shrikumar Suryanarayan, chairman at Sea6 Energy, and 3One4 Capital, are among other investors in the company. Anandkumar is now in talks to raise a Series-C round of about $30-40 million.
The bottomline is that “without foreign support, without the US government putting in money, UK government supporting us, we"d be dead," Anandkumar says. “For every hundred dollars I"ve raised for this company, hardly $2 has come from India."
“It"s a tough haul, but there is a pot of gold at the end of this rainbow and it won"t have gold coins. It"ll have many, many, many lives saved. That’s what is inspiring and what keeps us going like maniacs."
India is also in a unique position to completely change the world in drug discovery, just like in payment gateways and the digital stacks being built. India has got a “phenomenal database" with Aadhaar and CoWin (Covid-19 vaccination portal), for example. And entrepreneurs are emerging with deep scientific experience, Anandkumar says.
According to him, a combination of these entrepreneurs’ experience, AI, the digital stacks and “plus biology" will help India find affordable, and contextually valid solutions for its own problems of food, environment and health.